Scientists and dark sky advocates team up to flip the switch on light pollution

Residents of Fountain Hills, a small town near Phoenix, Arizona, fought against the night sky pollution to restore their Milky Way skies.
As a graduate student in observational astronomy at the University of Arizona during the 1970s, Diane Turnshek remembers the starry skies above the Kitt Peak National Observatory on the Tucson outskirts. Back then, she could observe faint objects like nebulae, galaxies, and star clusters on most nights.
When Turnshek moved to Pittsburgh in 1981, she found it almost impossible to see a clear night sky because the city’s countless lights created a bright dome of light called skyglow. Over the next two decades, Turnshek almost forgot what a dark sky looked like. She witnessed pristine dark skies in their full glory again during a visit to the Mars Desert Research Station in Utah in early 2000s.
“I was shocked at how beautiful the dark skies were in the West. That is when I realized that most parts of the world have lost access to starry skies because of light pollution,” says Turnshek, an astronomer and lecturer at Carnegie Mellon University. In 2015, she became a dark sky advocate.
Light pollution is defined as the excessive or wasteful use of artificial light.
Light-emitting diodes (LEDs) -- which became commercially available in 2002 and rapidly gained popularity in offices, schools, and hospitals when their price dropped six years later — inadvertently fueled the surge in light pollution. As traditional light sources like halogen, fluorescent, mercury, and sodium vapor lamps have been phased out or banned, LEDs became the main source of lighting globally in 2019. Switching to LEDs has been lauded as a win-win decision. Not only are they cheap but they also consume a fraction of electricity compared to their traditional counterparts.
But as cheap LED installations became omnipresent, they increased light pollution. “People have been installing LEDs thinking they are making a positive change for the environment. But LEDs are a lot brighter than traditional light sources,” explains Ashley Wilson, director of conservation at the International Dark-Sky Association (IDA). “Despite being energy-efficient, they are increasing our energy consumption. No one expected this kind of backlash from switching to LEDs.”
Light pollution impacts the circadian rhythms of all living beings — the natural internal process that regulates the sleep–wake cycle.
Currently, more than 80 percent of the world lives under light-polluted skies. In the U.S. and Europe, that figure is above 99 percent.
According to the IDA, $3 billion worth of electricity is lost to skyglow every year in the U.S. alone — thanks to unnecessary and poorly designed outdoor lighting installations. Worse, the resulting light pollution has insidious impacts on humans and wildlife — in more ways than one.
Disrupting the brain’s clock
Light pollution impacts the circadian rhythms of all living beings—the natural internal process that regulates the sleep–wake cycle. Humans and other mammals have neurons in their retina called intrinsically photosensitive retinal ganglion cells (ipRGCs). These cells collect information about the visual world and directly influence the brain’s biological clock in the hypothalamus.
The ipRGCs are particularly sensitive to the blue light that LEDs emit at high levels, resulting in suppression of melatonin, a hormone that helps us sleep. A 2020 JAMA Psychiatry study detailed how teenagers who lived in areas with bright outdoor lighting at night went to bed late and slept less, which made them more prone to mood disorders and anxiety.
“Many people are skeptical when they are told something as ubiquitous as lights could have such profound impacts on public health,” says Gena Glickman, director of the Chronobiology, Light and Sleep Lab at Uniformed Services University. “But when the clock in our brains gets exposed to blue light at nighttime, it could result in a lot of negative consequences like impaired cognitive function and neuro-endocrine disturbances.”
In the last 12 years, several studies indicated that light pollution exposure is associated with obesity and diabetes in humans and animals alike. While researchers are still trying to understand the exact underlying mechanisms, they found that even one night of too much light exposure could negatively affect the metabolic system. Studies have linked light pollution to a higher risk of hormone-sensitive cancers like breast and prostate cancer. A 2017 study found that female nurses exposed to light pollution have a 14 percent higher risk of breast cancer. The World Health Organization (WHO) identified long-term night shiftwork as a probable cause of cancer.
“We ignore our biological need for a natural light and dark cycle. Our patterns of light exposure have consequently become different from what nature intended,” explains Glickman.
Circadian lighting systems, designed to match individuals’ circadian rhythms, might help. The Lighting Research Center at Rensselaer Polytechnic Institute developed LED light systems that mimic natural lighting fluxes, required for better sleep. In the morning the lights shine brightly as does the sun. After sunset, the system dims, once again mimicking nature, which boosts melatonin production. It can even be programmed to increase blue light indoors when clouds block sunlight’s path through windows. Studies have shown that such systems might help reduce sleep fragmentation and cognitive decline. People who spend most of their day indoors can benefit from such circadian mimics.

When Diane Turnshek moved to Pittsburgh, she found it almost impossible to see a clear night sky because the city’s countless lights created a bright dome of light called skyglow.
Diane Turnshek
Leading to better LEDs
Light pollution disrupts the travels of millions of migratory birds that begin their long-distance journeys after sunset but end up entrapped within the sky glow of cities, becoming disoriented. A 2017 study in Nature found that nocturnal pollinators like bees, moths, fireflies and bats visit 62 percent fewer plants in areas with artificial lights compared to dark areas.
“On an evolutionary timescale, LEDs have triggered huge changes in the Earth’s environment within a relative blink of an eye,” says Wilson, the director of IDA. “Plants and animals cannot adapt so fast. They have to fight to survive with their existing traits and abilities.”
But not all types of LEDs are inherently bad -- it all comes down to how much blue light they emit. During the day, the sun emits blue light waves. By sunset, red and orange light waves become predominant, stimulating melatonin production. LED’s artificial blue light, when shining at night, disrupts that. For some unknown reason, there are more bluer color LEDs made and sold.
“Communities install blue color temperature LEDs rather than redder color temperature LEDs because more of the blue ones are made; they are the status quo on the market,” says Michelle Wooten, an assistant professor of astronomy at the University of Alabama at Birmingham.
Most artificial outdoor light produced is wasted as human eyes do not use them to navigate their surroundings.
While astronomers and the IDA have been educating LED manufacturers about these nuances, policymakers struggle to keep up with the growing industry. But there are things they can do—such as requiring LEDs to include dimmers. “Most LED installations can be dimmed down. We need to make the dimmable drivers a mandatory requirement while selling LED lighting,” says Nancy Clanton, a lighting engineer, designer, and dark sky advocate.
Some lighting companies have been developing more sophisticated LED lights that help support melatonin production. Lighting engineers at Crossroads LLC and Nichia Corporation have been working on creating LEDs that produce more light in the red range. “We live in a wonderful age of technology that has given us these new LED designs which cut out blue wavelengths entirely for dark-sky friendly lighting purposes,” says Wooten.
Dimming the lights to see better
The IDA and advocates like Turnshek propose that communities turn off unnecessary outdoor lights. According to the Department of Energy, 99 percent of artificial outdoor light produced is wasted as human eyes do not use them to navigate their surroundings.
In recent years, major cities like Chicago, Austin, and Philadelphia adopted the “Lights Out” initiative encouraging communities to turn off unnecessary lights during birds’ peak migration seasons for 10 days at a time. “This poses an important question: if people can live without some lights for 10 days, why can’t they keep them turned off all year round,” says Wilson.
Most communities globally believe that keeping bright outdoor lights on all night increases security and prevents crime. But in her studies of street lights’ brightness levels in different parts of the US — from Alaska to California to Washington — Clanton found that people felt safe and could see clearly even at low or dim lighting levels.
Clanton and colleagues installed LEDs in a Seattle suburb that provided only 25 percent of lighting levels compared to what they used previously. The residents reported far better visibility because the new LEDs did not produce glare. “Visual contrast matters a lot more than lighting levels,” Clanton says. Additionally, motion sensor LEDs for outdoor lighting can go a long way in reducing light pollution.
Flipping a switch to preserve starry nights
Clanton has helped draft laws to reduce light pollution in at least 17 U.S. states. However, poor awareness of light pollution led to inadequate enforcement of these laws. Also, getting thousands of counties and municipalities within any state to comply with these regulations is a Herculean task, Turnshek points out.
Fountain Hills, a small town near Phoenix, Arizona, has rid itself of light pollution since 2018, thanks to the community's efforts to preserve dark skies.
Until LEDs became mainstream, Fountain Hills enjoyed starry skies despite its proximity to Phoenix. A mountain surrounding the town blocks most of the skyglow from the city.
“Light pollution became an issue in Fountain Hills over the years because we were not taking new LED technologies into account. Our town’s lighting code was antiquated and out-of-date,” says Vicky Derksen, a resident who is also a part of the Fountain Hills Dark Sky Association founded in 2017. “To preserve dark skies, we had to work with the entire town to update the local lighting code and convince residents to follow responsible outdoor lighting practices.”
Derksen and her team first tackled light pollution in the town center which has a faux fountain in the middle of a lake. “The iconic centerpiece, from which Fountain Hills got its name, had the wrong types of lighting fixtures, which created a lot of glare,” adds Derksen. They then replaced several other municipal lighting fixtures with dark-sky-friendly LEDs.
The results were awe-inspiring. After a long time, residents could see the Milky Way with crystal clear clarity. Star-gazing activities made a strong comeback across the town. But keeping light pollution low requires constant work.
Derksen and other residents regularly measure artificial light levels in
Fountain Hills. Currently, the only major source of light pollution is from extremely bright, illuminated signs which local businesses had installed in different parts of the town. While Derksen says it is an uphill battle to educate local businesses about light pollution, Fountain Hills residents are determined to protect their dark skies.
“When a river gets polluted, it can take several years before clean-up efforts see any tangible results,” says Derksen. “But the effects are immediate when you work toward reducing light pollution. All it requires is flipping a switch.”
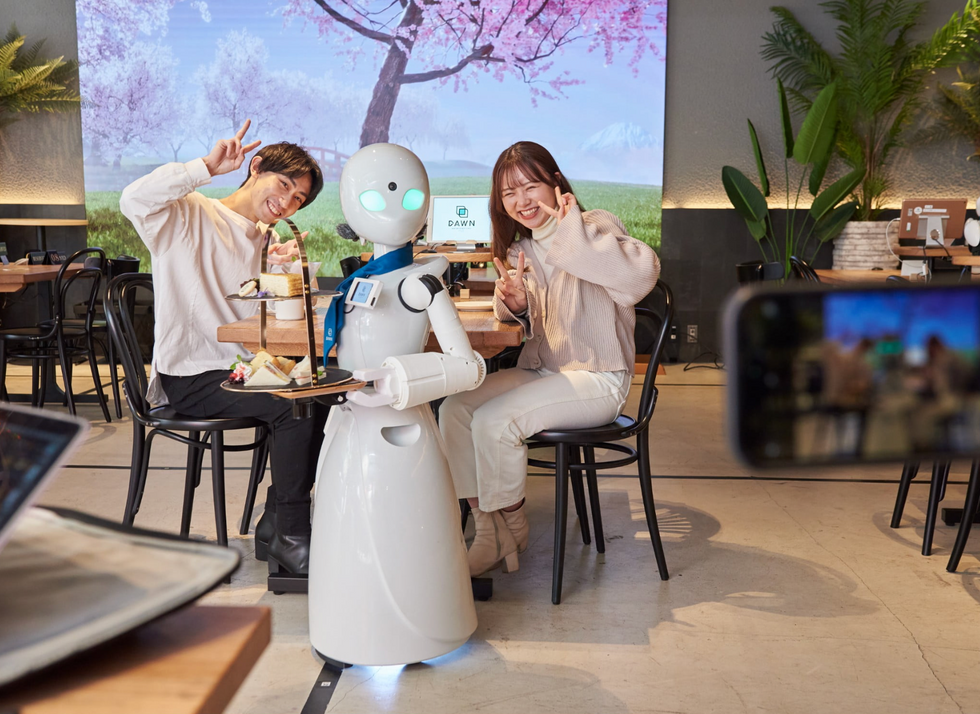
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
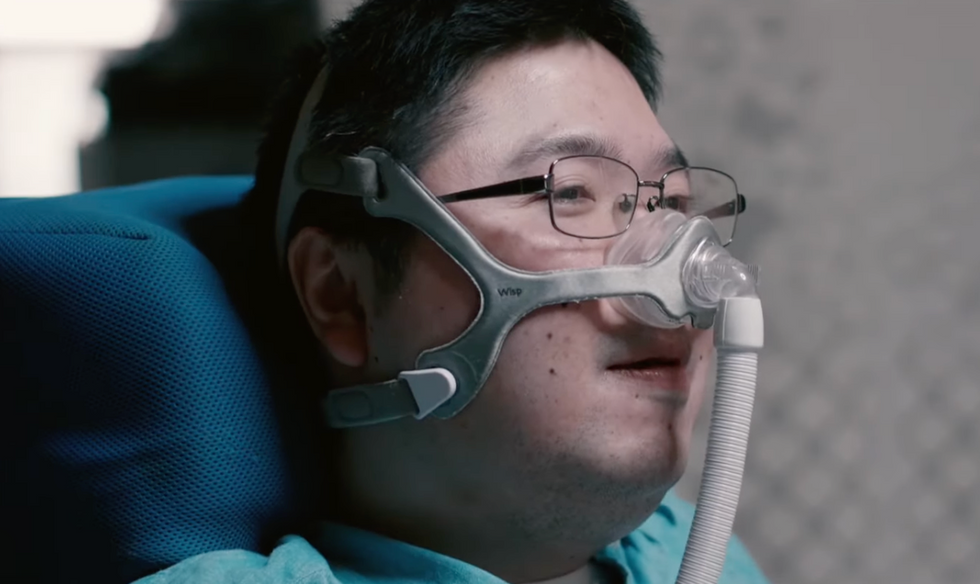
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.