“Coming Back from the Dead” Is No Longer Science Fiction

A man receiving cardiopulmonary resuscitation (CPR).
Last year, there were widespread reports of a 53-year-old Frenchman who had suffered a cardiac arrest and "died," but was then resuscitated back to life 18 hours after his heart had stopped.
The once black-and-white line between life and death is now blurrier than ever.
This was thought to have been possible in part because his body had progressively cooled down naturally after his heart had stopped, through exposure to the outside cold. The medical team who revived him were reported as being "stupefied" that they had been able to bring him back to life, in particular since he had not even suffered brain damage.
Interestingly, this man represents one of a growing number of extraordinary cases in which people who would otherwise be declared dead have now been revived. It is a testament to the incredible impact of resuscitation science -- a science that is providing opportunities to literally reverse death, and in doing so, shedding light on the age-old question of what happens when we die.
Death: Past and Present
Throughout history, the boundary between life and death was marked by the moment a person's heart stopped, breathing ceased, and brain function shut down. A person became motionless, lifeless, and was deemed irreversibly dead. This is because once the heart stops beating, blood flow stops and oxygen is cut off from all the body's organs, including the brain. Consequently, within seconds, breathing stops and brain activity comes to a halt. Since the cessation of the heart literally occurs in a "moment," the philosophical notion of a specific point in time of "irreversible" death still pervades society today. The law, for example, relies on "time of death," which corresponds to when the heart stops beating.
The advent of cardiopulmonary resuscitation (CPR) in the 1960s was revolutionary, demonstrating that the heart could potentially be restarted after it had stopped, and what had been a clear black-and-white line was shown to be potentially reversible in some people. What was once called death—the ultimate end point— was now widely called cardiac arrest, and became a starting point.
From then on, it was only if somebody had requested not to be resuscitated or when CPR was deemed to have failed that people would be declared dead by "cardiopulmonary criteria." Biologically, cardiac arrest and death by cardiopulmonary criteria are the same process, albeit marked at different points in time depending on when a declaration of death is made.
The apparent irreversibility of death as we know it may not necessarily reflect true irretrievable cellular damage inside the body.
Clearly, contrary to many people's perceptions, cardiac arrest is not a heart attack; it is the final step in death irrespective of cause, whether it be a stroke, a heart attack, a car accident, an overwhelming infection or cancer. This is how roughly 95 percent of the population are declared dead.
The only exception is the small proportion of people who may have suffered catastrophic brain injuries, but whose hearts can be artificially kept beating for a period of time on life-support machines. These people can be legally declared dead based on brain death criteria before their hearts have stopped. This is because the brain can die either from oxygen starvation after cardiac arrest or from massive trauma and internal bleeding. Either way, the brain dies hours or possibly longer after these injuries have taken place and not just minutes.
A Profound Realization
What has become increasingly clear is that the apparent irreversibility of death as we know it may not necessarily reflect true irretrievable cellular damage inside the body. This is consistent with a mounting understanding: it is only after a person actually dies that the cells in the body start to undergo their own process of death. Intriguingly, this process is something that can now be manipulated through medical intervention. Being cold is one of the factors that slows down the rate of cellular decay. The 53-year-old Frenchman's case and the other recent cases of resuscitation after prolonged periods of time illustrate this new understanding.
Last week's earth-shattering announcement by neuroscientist Dr. Nenad Sestan and his team out of Yale, published in the prestigious scientific journal Nature, provides further evidence that a time gap exists between actual death and cellular death in cadavers. In this seminal study, these researchers were able to restore partial function in pig brains four hours after their heads were severed from their bodies. These results follow from the pioneering work in 2001 of geneticist Fred Gage and colleagues from the Salk Institute, also published in Nature, which demonstrated the possibility of growing human brain cells in the laboratory by taking brain biopsies from cadavers in the mortuary up to 21 hours post-mortem.
The once black-and-white line between life and death is now blurrier than ever. Some people may argue this means these humans and pigs weren't truly "dead." However, that is like saying the people who were guillotined during the French Revolution were also not dead. Clearly, that is not the case. They were all dead. The problem is not death; it's our reliance on an outdated philosophical, rather than biological, notion of death.
Death can no longer be considered an absolute moment but rather a process that can be reversed even many hours after it has taken place.
But the distinction between irreversibility from a medical perspective and biological irreversibility may not matter much from a pragmatic perspective today. If medical interventions do not exist at any given time or place, then of course death cannot be reversed.
However, it is crucial to distinguish between biologically and medically: When "irreversible" loss of function arises due to inadequate treatment, then a person could be potentially brought back in the future when an alternative therapy becomes available, or even today if he or she dies in a location where novel treatments can slow down the rate of cell death. However, when true irreversible loss of function arises from a biological perspective, then no treatment will ever be able to reverse the process, whether today, tomorrow, or in a hundred years.
Probing the "Grey Zone"
Today, thanks to modern resuscitation science, death can no longer be considered an absolute moment but rather a process that can be reversed even many hours after it has taken place. How many hours? We don't really know.
One of the wider implications of our medical advances is that we can now study what happens to the human mind and consciousness after people enter the "grey zone," which marks the time after the heart stops, but before irreversible and irretrievable cell damage occurs, and people are then brought back to life. Millions have been successfully revived and many have reported experiencing a unique, universal, and transformative mental state.
Were they "dead"? Yes, according to all the criteria we have ever used. But they were able to be brought back before their "dead" bodies had reached the point of permanent, irreversible cellular damage. This reflects the period of death for all of us. So rather than a "near-death experience," I prefer a new terminology to describe these cases -- "an actual-death experience." These survivors' unique experiences are providing eyewitness testimonies of what we will all be likely to experience when we die.
Such an experience reportedly includes seeing a warm light, the presence of a compassionate perfect individual, deceased relatives, a review of their lives, a judgment of their actions and intentions as they pertain to their humanity, and in some cases a sensation of seeing doctors and nurses working to resuscitate them.
Are these experiences compatible with hallucinations or illusions? No -- in part, because these people have described real, verifiable events, which, by definition are not hallucinations, and in part, because their experiences are not compatible with confused and delirious memories that characterize oxygen deprivation.
The challenge for us scientifically is understanding how this is possible at a time when all our science tells us the brain shuts down.
For instance, it is hard to classify a structured meaningful review of one's life and one's humanity as hallucinatory or illusory. Instead, these experiences represent a new understanding of the overall human experience of death. As an intensive care unit physician for more than 10 years, I have seen numerous cases where these reports have been corroborated by my colleagues. In short, these survivors have been known to come back with reports of full consciousness, with lucid, well-structured thought processes and memory formation.
The challenge for us scientifically is understanding how this is possible at a time when all our science tells us the brain shuts down. The fact that these experiences occur is a paradox and suggests the undiscovered entity we call the "self," "consciousness," or "psyche" – the thing that makes us who we are - may not become annihilated at the point of so-called death.
At New York University, the State University of New York, and across 20 hospitals in the U.S. and Europe, we have brought together a new multi-disciplinary team of experts across many specialties, including neurology, cardiology, and intensive care. Together, we hope to improve cardiac arrest prevention and treatment, as well as to address the impact of new scientific discoveries on our understanding of what happens at death.
One of our first studies, Awareness during Resuscitation (AWARE), published in the medical journal Resuscitation in 2014, confirmed that some cardiac arrest patients report a perception of awareness without recall; others report detailed memories and experiences; and a few report full auditory and visual awareness and consciousness of their experience, from a time when brain function would be expected to have ceased.
While you probably have some opinion or belief about this based upon your own philosophical, religious, or cultural background, you may not realize that exploring what happens when we die is now a subject that science is beginning to investigate.
There is no question more intriguing to humankind. And for the first time in our history, we may finally uncover some real answers.
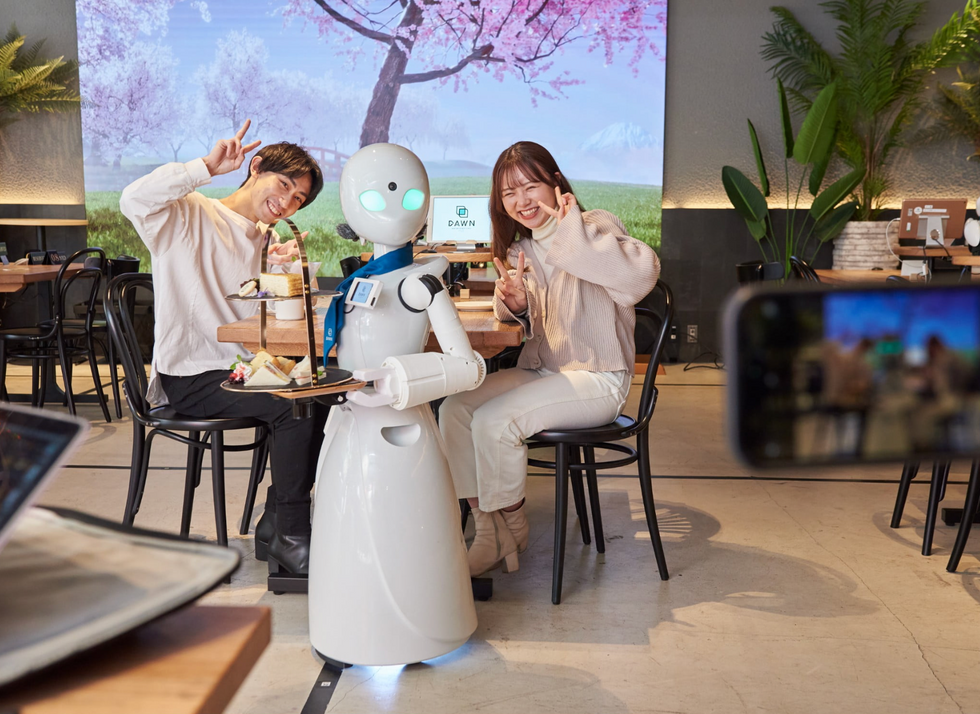
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.