As We Wait for a Vaccine, Scientists Work to Scale Up the Best COVID-19 Antibodies to Give New Patients
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
In this illustration of immune defense, Y-shaped proteins called antibodies attack a virus.
When we get sick, our immune system sends its soldier cells to the battlefield. Called B-cells, they "examine" the foreign particles that shouldn't be in our bloodstream—and start producing the antibodies, the proteins to neutralize the invaders.
To screen the antibodies, scientists have developed a proprietary way to make the effective ones glow – like a literal "lightbulb" moment.
The better these antibodies are at neutralizing the pathogen, the faster we recover.
The antibodies acquired from COVID-19 survivors already showed promise in treating other patients, but because they must be obtained from people, generating a regular supply is not feasible. To close the gap, researchers are trying to identify the B-cells that make the best antibodies—and then farm them in laboratories at scale.
Scientists at Berkley Lights, a biotechnology company in California, have been screening B-cells from recovered patients and testing the antibodies they release for virus-neutralizing abilities. To screen the antibodies, scientists there have developed a proprietary way to make the effective ones glow – like a literal "lightbulb" moment.
So how does it work? First, the individual B-cells are placed into microscopic chambers called nano-pens, where they secrete the antibodies. Once released, the antibodies are flushed over tiny beads that have bits of the viral particles attached to them, along with special molecules that can emit fluorescent light.
"When an antibody binds to the bead, we see a bright light on the bead," explains John Proctor, the company's senior vice president of antibody therapeutics. "So we can identify which cells are making the antibodies."
Then the antibodies are tested for their ability to counteract the coronavirus's spike proteins, which the virus uses to break into our cells. Not all antibodies are equally good at this crucial defense move—some can block only parts of the virus's machinery, while others can neutralize it completely. Proctor and his colleagues are looking for the latter.
Once scientists identify the best performing B-cells, they crack the cells open—or in scientific terms "lyse" them—and extract the genetic instructions for making the antibodies. As it turns out, B-cells aren't very efficient at pumping out massive amounts of antibodies, so scientists insert these genetic instructions into a different, more prolific type of cell.
Named Chinese Hamster Ovary Cells or CHO, these cells are commonly used in the pharmaceutical industry because they can generate therapeutic proteins en masse. Under the right nutrient conditions, which include a lot of sugar, CHO cells can keep making the antibodies at commercial levels. "They are engineered to operate in very large bioreactors," Proctor explains.
While traditional antibody screening can take three months, the Beacon System can do it in less than 24 hours.
Berkeley Lights' technology has already been used to screen the antibodies of recovered patients from Vanderbilt University Medical Center. In another example, a biotech company GenScript ProBio used the platform to screen mice engineered to have human antibodies for the coronavirus.
In addition to its small, lab-on-a-chip size, Berkeley Lights' system allows scientists to greatly speed up the screening process. While traditional antibody screening can take three months, the Beacon System can do it in less than 24 hours. "We only need one B-cell per pen and a couple of beads to see that fluorescent signal," Proctor says. "It is a more advanced way to process and analyze cells, and that level of sensitivity is unique to our technology."
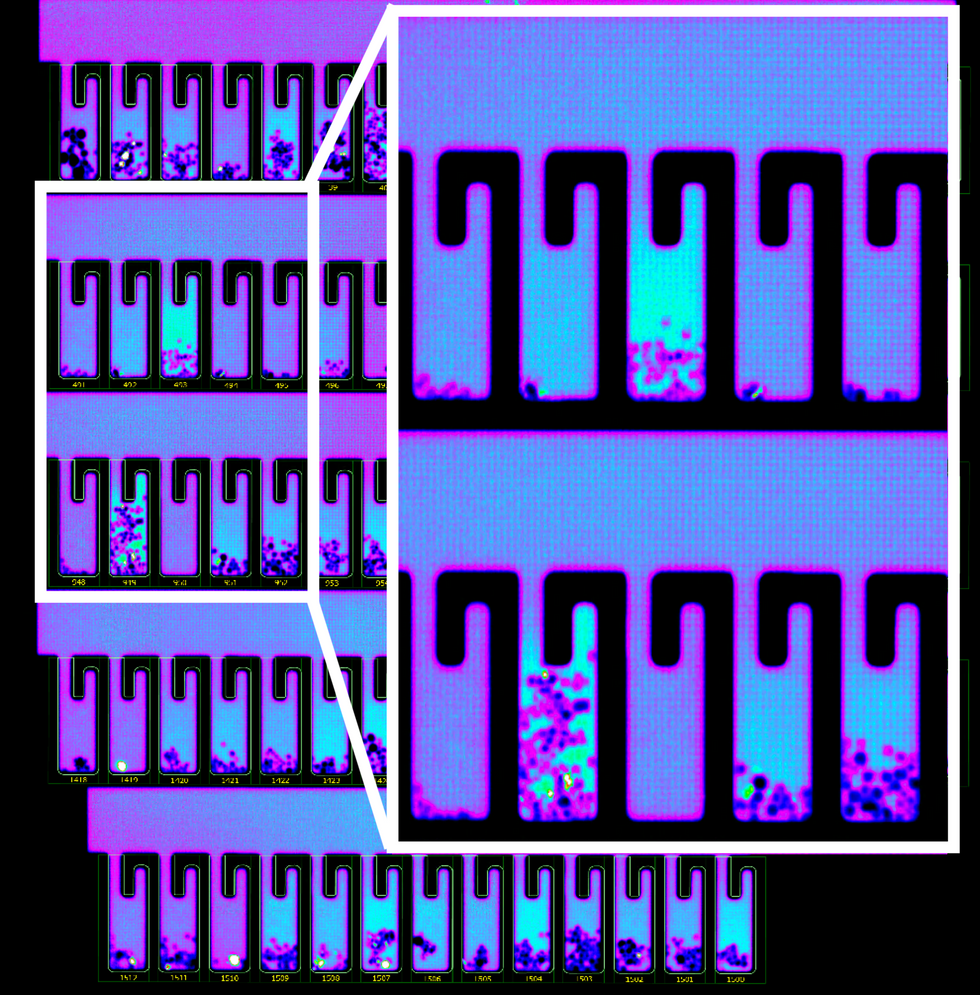
B-cells secreting antibodies inside the Berkeley Lights Beacon System Nano-Pens.
While vaccines are likely to take months to develop and test, antibodies might arrive to the battleground sooner. With the extremely limited treatment options for COVID-19, antibody-based therapeutics can potentially bridge this gap.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.
Urinary tract infections account for more than 8 million trips to the doctor each year.
Few things are more painful than a urinary tract infection (UTI). Common in men and women, these infections account for more than 8 million trips to the doctor each year and can cause an array of uncomfortable symptoms, from a burning feeling during urination to fever, vomiting, and chills. For an unlucky few, UTIs can be chronic—meaning that, despite treatment, they just keep coming back.
But new research, presented at the European Association of Urology (EAU) Congress in Paris this week, brings some hope to people who suffer from UTIs.
Clinicians from the Royal Berkshire Hospital presented the results of a long-term, nine-year clinical trial where 89 men and women who suffered from recurrent UTIs were given an oral vaccine called MV140, designed to prevent the infections. Every day for three months, the participants were given two sprays of the vaccine (flavored to taste like pineapple) and then followed over the course of nine years. Clinicians analyzed medical records and asked the study participants about symptoms to check whether any experienced UTIs or had any adverse reactions from taking the vaccine.
The results showed that across nine years, 48 of the participants (about 54%) remained completely infection-free. On average, the study participants remained infection free for 54.7 months—four and a half years.
“While we need to be pragmatic, this vaccine is a potential breakthrough in preventing UTIs and could offer a safe and effective alternative to conventional treatments,” said Gernot Bonita, Professor of Urology at the Alta Bro Medical Centre for Urology in Switzerland, who is also the EAU Chairman of Guidelines on Urological Infections.
The news comes as a relief not only for people who suffer chronic UTIs, but also to doctors who have seen an uptick in antibiotic-resistant UTIs in the past several years. Because UTIs usually require antibiotics, patients run the risk of developing a resistance to the antibiotics, making infections more difficult to treat. A preventative vaccine could mean less infections, less antibiotics, and less drug resistance overall.
“Many of our participants told us that having the vaccine restored their quality of life,” said Dr. Bob Yang, Consultant Urologist at the Royal Berkshire NHS Foundation Trust, who helped lead the research. “While we’re yet to look at the effect of this vaccine in different patient groups, this follow-up data suggests it could be a game-changer for UTI prevention if it’s offered widely, reducing the need for antibiotic treatments.”

