Are Physicians Morally Obligated to Prescribe Experimental Therapies?
A doctor reassuring a patient.
The federal 'Right to Try' bill in the United States recently passed the House and requires Senate approval before it becomes law. The bill would provide patients access to experimental drugs and other products that have not received approval from the Food and Drug Administration (FDA), including stem cell treatments.
It's not enough to act on a hunch that it might work.
Most folks think this is a good thing, but several express concern over whether the law would truly help patients. Even if a company allows patients to access an experimental drug, an important question remains: Should a doctor prescribe it?
Before such a drug can be prescribed, the federal bill states that a physician must "certify" that the patient has exhausted all available treatments or does not meet the criteria for standard treatment. Even after determining eligibility, a physician needs to consider a few points first. It's not enough to act on a hunch that it might work. The concept of medical innovation could help doctors figure out if prescribing an experimental treatment is the right thing to do.
Medical innovation falls within the doctor's scope of practice. Based on their experience and sound scientific rationale, physicians can "innovate" and offer treatment tailored to a patient with the goal of improving health. This differs from the goal of clinical research, which is to produce generalizable knowledge, not necessarily to benefit patients. In medical specialties like surgery, many of the standard procedures were developed through medical innovation, not clinical trials. Under the 'Right to Try,' a physician could ethically prescribe an experimental therapy as medical innovation if the following conditions are met.
Medical innovation should follow similar ethical and scientific oversight as clinical research.
First, there must be sound scientific rationale, and evidence of safety and efficacy of the innovative treatment from preclinical (animal and lab) research or clinical (human) research. The 'Right to Try' bill permits access to experimental products only after safety is demonstrated from a phase 1 clinical trial. This initial testing, called "first in human," aims to determine safety and dosing of an experimental product on typically around 20 to 100 people who are healthy volunteers or have a condition. This way, a physician can be assured that there is some evidence indicating the product is safe.
Efficacy must be demonstrated in animal and lab preclinical studies in order to gain permission from the FDA to do a phase 1 trial in the first place. This way, a doctor can also be assured that sound scientific rationale exists indicating a potential benefit to the patient. Only through further phase 2 and 3 clinical trials on hundreds or more people would a doctor know with greater certainty that the therapy works, but this might take many more years.
A doctor should not completely rely on what others in the scientific community think about the experimental treatment and should have appropriate expertise. This includes knowledge about the disease, familiarity with treating such patients, and an understanding of how the experimental treatment works, including administering it.
Second, medical innovation should follow similar ethical and scientific oversight as clinical research. Physicians should write a protocol for administering the experimental therapy and have it reviewed by clinical, scientific, and ethics experts at their institution. A protocol would include all the information on how the doctor would provide the therapy to patients, including dosages, monitoring, what happens if there are side effects, and much more. The experts would examine various components of the plan, look at informed consent, and ensure a favorable benefit-to-risk ratio, among other aspects.
When weighing whether to prescribe an experimental treatment, doctors need to base this decision on sound science and relevant clinical experience, not on hope or desperation.
Third, doctors should properly inform their patients about the risks (including if the risks are unknown), possible benefits, and the details of the procedure to be undertaken, and they must obtain the patient's consent.
Fourth, physicians should thoroughly monitor and diligently document all aspects of the outcomes of the procedure, various clinical indicators, and adverse events. During the course of providing an experimental therapy, if harm to a patient occurs, the physician is obligated to alter the course of the treatment or stop it. Similarly, if evidence from an ongoing clinical trial shows that the experimental treatment might help some but not all patients, the doctor needs to modify the plan accordingly.
Fifth, upon completing the experimental treatment, physicians should publish their findings to share the knowledge. Note that medical innovation is not meant to replace clinical trials. The two can be complementary, and medical innovation can lead to the design of clinical trials to demonstrate safety and efficacy.
Other experts may not agree that it can be ethical for a physician to prescribe an unapproved drug. Such dissenters would claim that physicians should only prescribe medications when there is substantial scientific and clinical certainty that a product is safe and effective for patients. They are also likely to oppose most forms of medical innovation. Yet even after undergoing rigorous clinical trials, some approved products have been shown to be unsafe or ineffective and are removed from the market.
While it seems that more evidence is better, doctors need to be mindful that patients are suffering and some may never receive access to drugs still in the pipeline. Bound by the Hippocratic Oath – the main tenet being "do no harm" – doctors are obligated to prescribe therapies that will help their patients. When weighing whether to prescribe an experimental treatment, doctors need to base this decision on sound science and relevant clinical experience, not on hope or desperation. Given that patients who want to participate in the 'Right to Try' movement have exhausted all other options and their condition may be worsening, it would seem ethically appropriate for a physician to treat them with an experimental drug, as long as the criteria listed above are satisfied.
The views expressed are the author's personal views, and do not necessarily reflect the policy or position of Mayo Clinic.
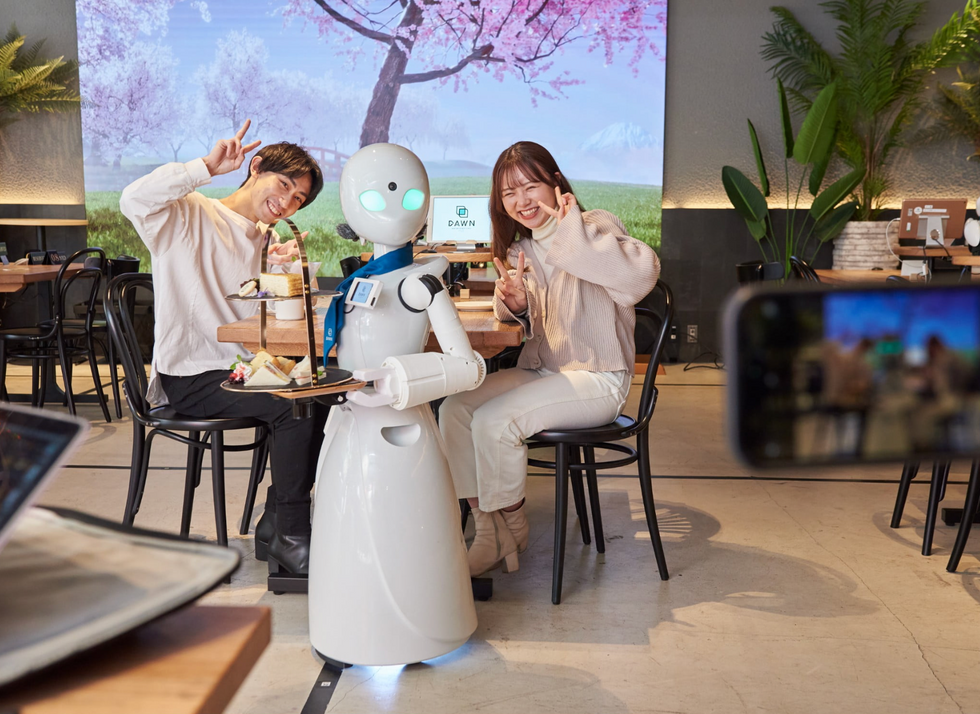
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
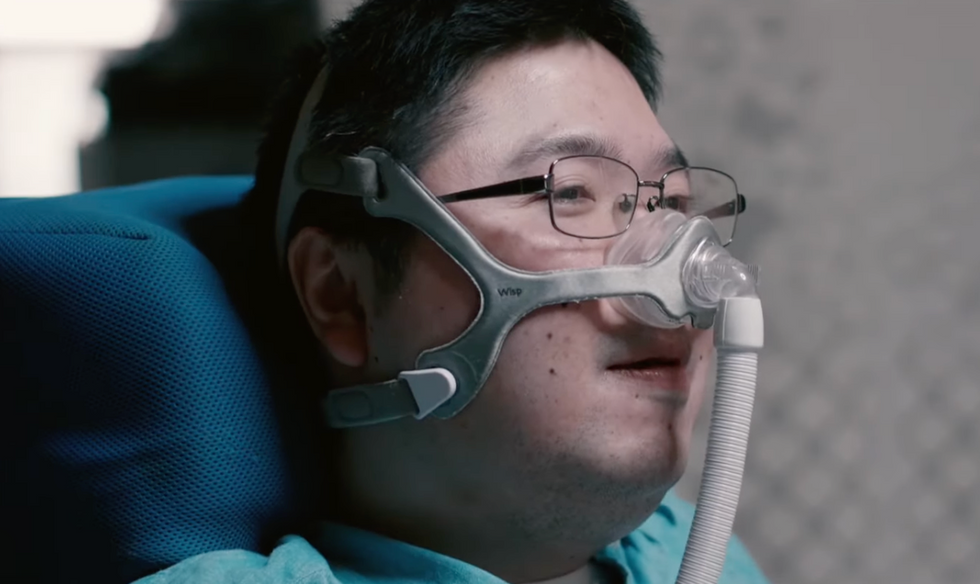
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.