Anti-Aging Pioneer Aubrey de Grey: “People in Middle Age Now Have a Fair Chance”
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

Aubrey de Grey at the World Stem Cell Summit in Miami on January 25, 2018.
Aging is not a mystery, says famed researcher Dr. Aubrey de Grey, perhaps the world's foremost advocate of the provocative view that medical technology will one day allow humans to control the aging process and live healthily into our hundreds—or even thousands.
"The cultural attitudes toward all of this are going to be completely turned upside down by sufficiently promising results in the lab, in mice."
He likens aging to a car wearing down over time; as the body operates normally, it accumulates damage which can be tolerated for a while, but eventually sends us into steep decline. The most promising way to escape this biological reality, he says, is to repair the damage as needed with precise scientific tools.
The bad news is that doing this groundbreaking research takes a long time and a lot of money, which has not always been readily available, in part due to a cultural phenomenon he terms "the pro-aging trance." Cultural attitudes have long been fatalistic about the inevitability of aging; many people balk at the seemingly implausible prospect of indefinite longevity.
But the good news for de Grey—and those who are cheering him on—is that his view is becoming less radical these days. Both the academic and private sectors are racing to tackle aging; his own SENS Research Foundation, for one, has spun out into five different companies. Defeating aging, he says, "is not just a future industry; it's an industry now that will be both profitable and extremely good for your health."
De Grey sat down with Editor-in-Chief Kira Peikoff at the World Stem Cell Summit in Miami to give LeapsMag the latest scoop on his work. Here is an edited and condensed version of our conversation.
Since your book Ending Aging was published a decade ago, scientific breakthroughs in stem cell research, genome editing, and other fields have taken the world by storm. Which of these have most affected your research?
They have all affected it a lot in one way, and hardly at all in another way. They have speeded it up--facilitated short cuts, ways to get where we're already trying to go. What they have not done is identified any fundamental changes to the overall strategy. In the book, we described the seven major types of damage, and particular ways of going about fixing each of them, and that hasn't changed.
"Repair at the microscopic level, one would be able to expect to do without surgery, just by injecting the right kind of stem cells."
Has any breakthrough specifically made the biggest impact?
It's not just the obvious things, like iPS (induced pluripotent stem cells) and CRISPR (a precise tool for editing genes). It's also the more esoteric things that applied specifically to certain of our areas, but most people don't really know about them. For example, the identification of how to control something called co-translational mitochondrial protein import.
How much of the future of anti-aging treatments will involve regeneration of old tissue, or wholesale growth of new organs?
The more large-scale ones, regenerating whole new organs, are probably only going to play a role in the short-term and will be phased out relatively rapidly, simply because, in order to be useful, one has to employ surgery, which is really invasive. We'll want to try to get around that, but it seems quite likely that in the very early stages, the techniques we have for repairing things at the molecular and cellular level in situ will be insufficiently comprehensive, and so we will need to do the more sledgehammer approach of building a whole new organ and sticking it in.
Every time you are in a position where you're replacing an organ, you have the option, in principle, of repairing the organ, without replacing it. And repair at the microscopic level, one would be able to expect to do without surgery, just by injecting the right kind of stem cells or whatever. That would be something one would expect to be able to apply to someone much closer to death's door and much more safely in general, and probably much more cheaply. One would expect that subsequent generations of these therapies would move in that direction.
Your foundation is working on an initiative requiring $50 million in funding—
Well, if we had $50 million per year in funding, we could go about three times faster than we are on $5 million per year.
And you're looking at a 2021 timeframe to start human trials?
That's approximate. Remember, because we accumulate in the body so many different types of damage, that means we have many different types of therapy to repair that damage. And of course, each of those types has to be developed independently. It's very much a divide and conquer therapy. The therapies interact with each other to some extent; the repair of one type of damage may slow down the creation of another type of damage, but still that's how it's going to be.
And some of these therapies are much easier to implement than others. The easier components of what we need to do are already in clinical trials—stem cell therapies especially, and immunotherapy against amyloid in the brain, for example. Even in phase III clinical trials in some cases. So when I talk about a timeframe like 2021, or early 20s shall we say, I'm really talking about the most difficult components.
What recent strides are you most excited about?
Looking back over the past couple of years, I'm particularly proud of the successes we've had in the very most difficult areas. If you go through the 7 components of SENS, there are two that have absolutely been stuck in a rut and have gotten nowhere for 15 to 20 years, and we basically fixed that in both cases. We published two years ago in Science magazine that essentially showed a way forward against the stiffening of the extracellular matrix, which is responsible for things like wrinkles and hypertension. And then a year ago, we published a real breakthrough paper with regard to placing copies of the mitochondria DNA in the nuclear DNA modified in such a way that they still work, which is an idea that had been around for 30 years; everyone had given up on it, some a long time ago, and we basically revived it.
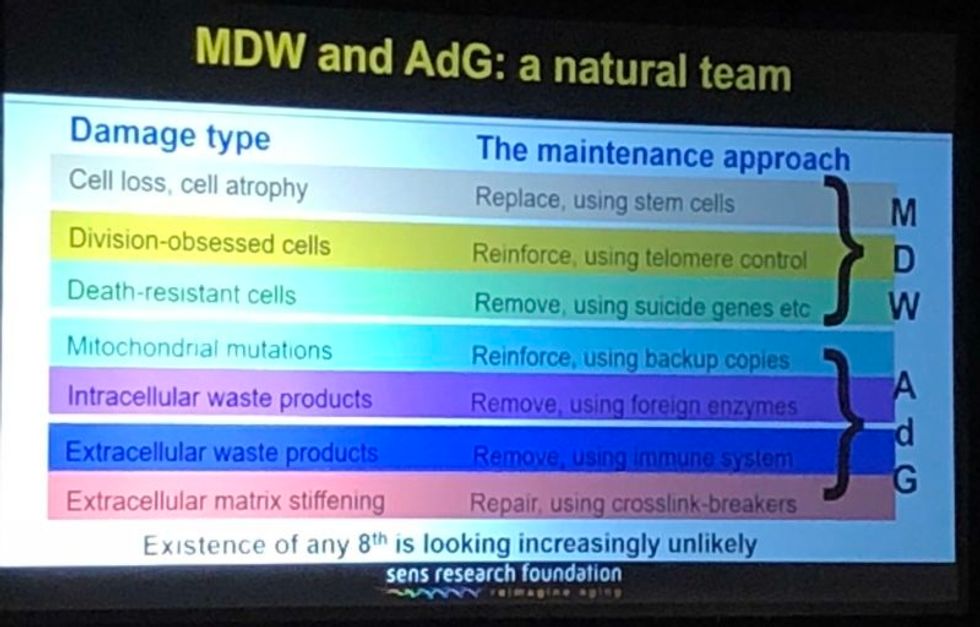
A slide presented by Aubrey de Grey, referencing his collaboration with Mike West at AgeX, showing the 7 types of damage that he believes must be repaired to end aging.
(Courtesy Kira Peikoff)
That's exciting. What do you think are the biggest barriers to defeating aging today: the technological challenges, the regulatory framework, the cost, or the cultural attitude of the "pro-aging" trance?
One can't really address those independently of each other. The technological side is one thing; it's hard, but we know where we're going, we've got a plan. The other ones are very intertwined with each other. A lot of people are inclined to say, the regulatory hurdle will be completely insurmountable, plus people don't recognize aging as a disease, so it's going to be a complete nonstarter. I think that's nonsense. And the reason is because the cultural attitudes toward all of this are going to be completely turned upside down before we have to worry about the regulatory hurdles. In other words, they're going to be turned upside down by sufficiently promising results in the lab, in mice. Once we get to be able to rejuvenate actually old mice really well so they live substantially longer than they otherwise would have done, in a healthy state, everyone's going to know about it and everyone's going to demand – it's not going to be possible to get re-elected unless you have a manifesto commitment to turn the FDA completely upside down and make sure this happens without any kind of regulatory obstacle.
I've been struggling away all these years trying to bring little bits of money in the door, and the reason I have is because of the skepticism as to regards whether this could actually work, combined with the pro-aging trance, which is a product of the skepticism – people not wanting to get their hopes up, so finding excuses about aging being a blessing in disguise, so they don't have to think about it. All of that will literally disintegrate pretty much overnight when we have the right kind of sufficiently impressive progress in the lab. Therefore, the availability of money will also [open up]. It's already cracking: we're already seeing the beginnings of the actual rejuvenation biotechnology industry that I've been talking about with a twinkle in my eye for some years.
"For humans, a 50-50 chance would be twenty years at this point, and there's a 10 percent chance that we won't get there for a hundred years."
Why do you think the culture is starting to shift?
There's no one thing yet. There will be that tipping point I mentioned, perhaps five years from now when we get a real breakthrough, decisive results in mice that make it simply impossible to carry on being fatalistic about all this. Prior to that, what we're already seeing is the impact of sheer old-school repeat advertising—me going out there, banging away and saying the same fucking thing again and again, and nobody saying anything that persuasively knocks me down. … And it's also the fact that we are making incremental amounts of progress, not just ourselves, but the scientific community generally. It has become incrementally more plausible that what I say might be true.
I'm sure you hate getting the timeline question, but if we're five years away from this breakthrough in mice, it's hard to resist asking—how far is that in terms of a human cure?
When I give any kind of timeframes, the only real care I have to take is to emphasize the variance. In this case I think we have got a 50-50 chance of getting to that tipping point in mice within five years from now, certainly it could be 10 or 15 years if we get unlucky. Similarly, for humans, a 50-50 chance would be twenty years at this point, and there's a 10 percent chance that we won't get there for a hundred years.
"I don't get people coming to me saying, well I don't think medicine for the elderly should be done because if it worked it would be a bad thing. People like to ignore this contradiction."
What would you tell skeptical people are the biggest benefits of a very long-lived population?
Any question about the longevity of people is the wrong question. Because the longevity that people fixate about so much will only ever occur as a side effect of health. However long ago you were born or however recently, if you're sick, you're likely to die fairly soon unless we can stop you being sick. Whereas if you're healthy, you're not. So if we do as well as we think we can do in terms of keeping people healthy and youthful however long ago they were born, then the side effect in terms of longevity and life expectancy is likely to be very large. But it's still a side effect, so the way that people actually ought to be—in fact have a requirement to be—thinking, is about whether they want people to be healthy.
Now I don't get people coming to me saying, well I don't think medicine for the elderly should be done because if it worked it would be a bad thing. People like to ignore this contradiction, they like to sweep it under the carpet and say, oh yeah, aging is totally a good thing.
People will never actually admit to the fact that what they are fundamentally saying is medicine for the elderly, if it actually works, would be bad, but still that is what they are saying.
Shifting gears a bit, I'm curious to find out which other radical visionaries in science and tech today you most admire?
Fair question. One is Mike West. I have the great privilege that I now work for him part-time with Age X. I have looked up to him very much for the past ten years, because what he did over the past 20 years starting with Geron is unimaginable today. He was working in an environment where I would not have dreamt of the possibility of getting any private money, any actual investment, in something that far out, that far ahead of its time, and he did it, again and again. It's insane what he managed to do.
What about someone like Elon Musk?
Sure, he's another one. He is totally impervious to the caution and criticism and conservatism that pervades humanity, and he's getting on making these bloody self-driving cars, space tourism, and so on, making them happen. He's thinking just the way I'm thinking really.
"You can just choose how frequently and how thoroughly you repair the damage. And you can make a different choice next time."
You famously said ten years ago that you think the first person to live to 1000 is already alive. Do you think that's still the case?
Definitely, yeah. I can't see how it could not be. Again, it's a probabilistic thing. I said there's at least a 10 percent chance that we won't get to what I call Longevity Escape Velocity for 100 years and if that's true, then the statement about 1000 years being alive already is not going to be the case. But for sure, I believe that the beneficiaries of what we may as well call SENS 1.0, the point where we get to LEV, those people are exceptionally unlikely ever to suffer from any kind of ill health correlated with their age. Because we will never fall below Longevity Escape Velocity once we attain it.
Could someone who was just born today expect—
I would say people in middle age now have a fair chance. Remember – a 50/50 chance of getting to LEV within 20 years, and when you get there, you don't just stay at biologically 70 or 80, you are rejuvenated back to biologically 30 or 40 and you stay there, so your risk of death each year is not related to how long ago you were born, it's the same as a young adult. Today, that's less than 1 in 1000 per year, and that number is going to go down as we get self-driving cars and all that, so actually 1000 is a very conservative number.
So you would be able to choose what age you wanted to go back to?
Oh sure, of course, it's just like a car. What you're doing is you're repairing damage, and the damage is still being created by the body's metabolism, so you can just choose how frequently and how thoroughly you repair the damage. And you can make a different choice next time.
What would be your perfect age?
I have no idea. That's something I don't have an opinion about, because I could change it whenever I like.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.
Urinary tract infections account for more than 8 million trips to the doctor each year.
Few things are more painful than a urinary tract infection (UTI). Common in men and women, these infections account for more than 8 million trips to the doctor each year and can cause an array of uncomfortable symptoms, from a burning feeling during urination to fever, vomiting, and chills. For an unlucky few, UTIs can be chronic—meaning that, despite treatment, they just keep coming back.
But new research, presented at the European Association of Urology (EAU) Congress in Paris this week, brings some hope to people who suffer from UTIs.
Clinicians from the Royal Berkshire Hospital presented the results of a long-term, nine-year clinical trial where 89 men and women who suffered from recurrent UTIs were given an oral vaccine called MV140, designed to prevent the infections. Every day for three months, the participants were given two sprays of the vaccine (flavored to taste like pineapple) and then followed over the course of nine years. Clinicians analyzed medical records and asked the study participants about symptoms to check whether any experienced UTIs or had any adverse reactions from taking the vaccine.
The results showed that across nine years, 48 of the participants (about 54%) remained completely infection-free. On average, the study participants remained infection free for 54.7 months—four and a half years.
“While we need to be pragmatic, this vaccine is a potential breakthrough in preventing UTIs and could offer a safe and effective alternative to conventional treatments,” said Gernot Bonita, Professor of Urology at the Alta Bro Medical Centre for Urology in Switzerland, who is also the EAU Chairman of Guidelines on Urological Infections.
The news comes as a relief not only for people who suffer chronic UTIs, but also to doctors who have seen an uptick in antibiotic-resistant UTIs in the past several years. Because UTIs usually require antibiotics, patients run the risk of developing a resistance to the antibiotics, making infections more difficult to treat. A preventative vaccine could mean less infections, less antibiotics, and less drug resistance overall.
“Many of our participants told us that having the vaccine restored their quality of life,” said Dr. Bob Yang, Consultant Urologist at the Royal Berkshire NHS Foundation Trust, who helped lead the research. “While we’re yet to look at the effect of this vaccine in different patient groups, this follow-up data suggests it could be a game-changer for UTI prevention if it’s offered widely, reducing the need for antibiotic treatments.”

