Top Fertility Doctor: Artificially Created Sperm and Eggs "Will Become Normal" One Day
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
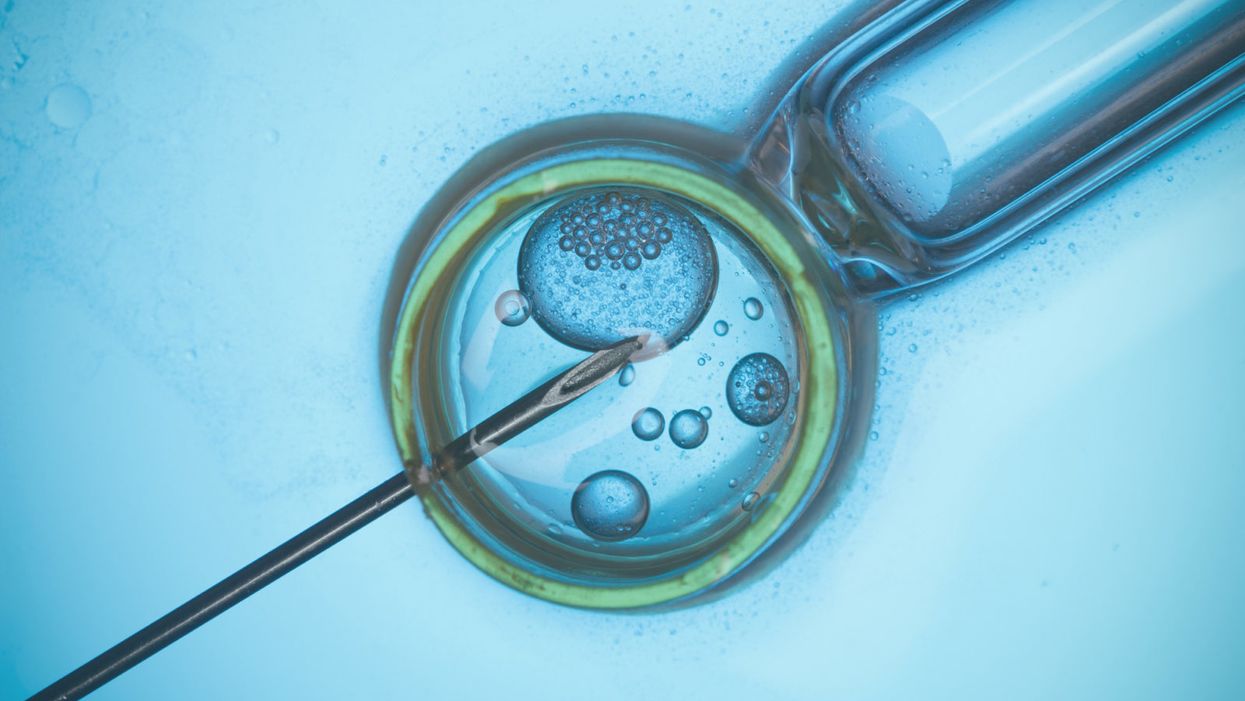
A rendering of in vitro fertilization.
Imagine two men making a baby. Or two women. Or an infertile couple. Or an older woman whose eggs are no longer viable. None of these people could have a baby today without the help of an egg or sperm donor.
Cells scraped from the inside of your cheek could one day be manipulated to become either eggs or sperm.
But in the future, it may be possible for them to reproduce using only their own genetic material, thanks to an emerging technology called IVG, or in vitro gametogenesis.
Researchers are learning how to reprogram adult human cells like skin cells to become lab-created egg and sperm cells, which could then be joined to form an embryo. In other words, cells scraped from the inside of your cheek could one day be manipulated to become either eggs or sperm, no matter your gender or your reproductive fitness.
In 2016, Japanese scientists proved that the concept could be successfully carried out in mice. Now some experts, like Dr. John Zhang, the founder and CEO of New Hope Fertility Center in Manhattan, say it's just "a matter of time" before the method is also made to work in humans.
Such a technological tour de force would upend our most basic assumptions about human reproduction and biology. Combined with techniques like gene editing, these tools could eventually enable prospective parents to have an unprecedented level of choice and control over their children's origins. It's a wildly controversial notion, and an especially timely one now that a Chinese scientist has announced the birth of the first allegedly CRISPR-edited babies. (The claims remain unverified.)
Zhang himself is no stranger to controversy. In 2016, he stunned the world when he announced the birth of a baby conceived using the DNA of three people, a landmark procedure intended to prevent the baby from inheriting a devastating neurological disease. (Zhang went to a clinic in Mexico to carry out the procedure because it is prohibited in the U.S.) Zhang's other achievements to date include helping a 49-year-old woman have a baby using her own eggs and restoring a young woman's fertility through an ovarian tissue transplant surgery.
Zhang recently sat down with our Editor-in-Chief in his New York office overlooking Columbus Circle to discuss the fertility world's latest provocative developments. Here are his top ten insights:
Clearly [gene-editing embryos] will be beneficial to mankind, but it's a matter of how and when the work is done.
1) On a Chinese scientist's claim of creating the first CRISPR-edited babies:
I'm glad that we made a first move toward a clinical application of this technology for mankind. Somebody has to do this. Whether this was a good case or not, there is still time to find out.
Clearly it will be beneficial to mankind, but it's a matter of how and when the work is done. Like any scientific advance, it has to be done in a very responsible way.
Today's response is identical to when the world's first IVF baby was announced in 1978. The major news media didn't take it seriously and thought it was evil, wanted to keep a distance from IVF. Many countries even abandoned IVF, but today you see it is a normal practice. And it took almost 40 years [for the researchers] to win a Nobel Prize.
I think we need more time to understand how this work was done medically, ethically, and let the scientist have the opportunity to present how it was done and let a scientific journal publish the paper. Before these become available, I don't think we should start being upset, scared, or giving harsh criticism.
2) On the international outcry in response to the news:
I feel we are in scientific shock, with many thinking it came too fast, too soon. We all embrace modern technology, but when something really comes along, we fear it. In an old Chinese saying, one of the masters always dreamed of seeing the dragon, and when the dragon really came, he got scared.

Dr. John Zhang, the founder and CEO of New Hope Fertility Center in Manhattan, pictured in his office.
3) On the Western world's perception that Chinese scientists sometimes appear to discount ethics in favor of speedy breakthroughs:
I think this perception is not fair. I don't think China is very casual. It's absolutely not what people think. I don't want people to feel that this case [of CRISPR-edited babies] will mean China has less standards over how human reproduction should be performed. Just because this happened, it doesn't mean in China you can do anything you want.
As far as the regulation of IVF clinics, China is probably the most strictly regulated of any country I know in this world.
4) On China's first public opinion poll gauging attitudes toward gene-edited babies, indicating that more than 60 percent of survey respondents supported using the technology to prevent inherited diseases, but not to enhance traits:
There is a sharp contrast between the general public and the professional world. Being a working health professional and an advocate of scientists working in this field, it is very important to be ethically responsible for what we are doing, but my own feeling is that from time to time we may not take into consideration what the patient needs.
5) On how the three-parent baby is doing today, several years after his birth:
No news is good news.
6) On the potentially game-changing research to develop artificial sperm and eggs:
First of all I think that anything that's technically possible, as long as you are not harmful to other people, to other societies, as long as you do it responsibly, and this is a legitimate desire, I think eventually it will become reality.
My research for now is really to try to overcome the very next obstacle in our field, which is how to let a lady age 44 or older have a baby with her own genetic material.
Practically 99 percent of women over age 43 will never make a baby on their own. And after age 47, we usually don't offer donor egg IVF anymore.
But with improved longevity, and quality of life, the lifespan of females continues to increase. In Japan, the average for females is about 89 years old. So for more than half of your life, you will not be able to produce a baby, which is quite significant in the animal kingdom. In most of the animal kingdom, their reproductive life is very much the same as their life, but then you can argue in the animal kingdom unlike a human being, it doesn't take such a long time for them to contribute to the society because once you know how to hunt and look for food, you're done.
"I think this will become a major ethical debate: whether we should let an older lady have a baby at a very late state of her life."
But humans are different. You need to go to college, get certain skills. It takes 20 years to really bring a human being up to become useful to society. That's why the mom and dad are not supposed to have the same reproductive life equal to their real life.
I think this will become a major ethical debate: whether we should let an older lady have a baby at a very late state of her life and leave the future generation in a very vulnerable situation in which they may lack warm caring, proper guidance, and proper education.
7) On using artificial gametes to grant more reproductive choices to gays and lesbians:
I think it is totally possible to have two sperm make a baby, and two eggs make babies.
If we have two guys, one guy to produce eggs, or two girls, one would have to become sperm. Basically you are creating artificial gametes or converting with gametes from sperm to become egg or egg to become a sperm. Which may not necessarily be very difficult. The key is to be able to do nuclear reprogramming.
So why can two sperm not make offspring now? You get exactly half of your genes from each parent. The genes have their own imprinting that say "made in mom," "made in dad." The two sperm would say "made in dad," "made in dad." If I can erase the "made in dad," and say "made in mom," then these sperm can make offspring.
8) On how close science is to creating artificial gametes for clinical use in pregnancies:
It's very hard to say until we accomplish it. It could be very quick. It could be it takes a long time. I don't want to speculate.
"I think these technologies are the solid foundation just like when we designed the computer -- we never thought a computer would become the iPhone."
9) On whether there should be ethical red lines drawn by authorities or whether the decisions should be left to patients and scientists:
I think we cannot believe a hundred percent in the scientist and the patient but it should not be 100 percent authority. It should be coming from the whole of society.
10) On his expectations for the future:
We are living in a very exciting world. I think that all these technologies can really change the way of mankind and also are not just for baby-making. The research, the experience, the mechanism we learn from these technologies, they will shine some great lights into our long-held dream of being a healthy population that is cancer-free and lives a long life, let's say 120 years.
I think these technologies are the solid foundation just like when we designed the computer -- we never thought a computer would become the iPhone. Imagine making a computer 30 years ago, that this little chip will change your life.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.
Urinary tract infections account for more than 8 million trips to the doctor each year.
Few things are more painful than a urinary tract infection (UTI). Common in men and women, these infections account for more than 8 million trips to the doctor each year and can cause an array of uncomfortable symptoms, from a burning feeling during urination to fever, vomiting, and chills. For an unlucky few, UTIs can be chronic—meaning that, despite treatment, they just keep coming back.
But new research, presented at the European Association of Urology (EAU) Congress in Paris this week, brings some hope to people who suffer from UTIs.
Clinicians from the Royal Berkshire Hospital presented the results of a long-term, nine-year clinical trial where 89 men and women who suffered from recurrent UTIs were given an oral vaccine called MV140, designed to prevent the infections. Every day for three months, the participants were given two sprays of the vaccine (flavored to taste like pineapple) and then followed over the course of nine years. Clinicians analyzed medical records and asked the study participants about symptoms to check whether any experienced UTIs or had any adverse reactions from taking the vaccine.
The results showed that across nine years, 48 of the participants (about 54%) remained completely infection-free. On average, the study participants remained infection free for 54.7 months—four and a half years.
“While we need to be pragmatic, this vaccine is a potential breakthrough in preventing UTIs and could offer a safe and effective alternative to conventional treatments,” said Gernot Bonita, Professor of Urology at the Alta Bro Medical Centre for Urology in Switzerland, who is also the EAU Chairman of Guidelines on Urological Infections.
The news comes as a relief not only for people who suffer chronic UTIs, but also to doctors who have seen an uptick in antibiotic-resistant UTIs in the past several years. Because UTIs usually require antibiotics, patients run the risk of developing a resistance to the antibiotics, making infections more difficult to treat. A preventative vaccine could mean less infections, less antibiotics, and less drug resistance overall.
“Many of our participants told us that having the vaccine restored their quality of life,” said Dr. Bob Yang, Consultant Urologist at the Royal Berkshire NHS Foundation Trust, who helped lead the research. “While we’re yet to look at the effect of this vaccine in different patient groups, this follow-up data suggests it could be a game-changer for UTI prevention if it’s offered widely, reducing the need for antibiotic treatments.”

