Is a Successful HIV Vaccine Finally on the Horizon?
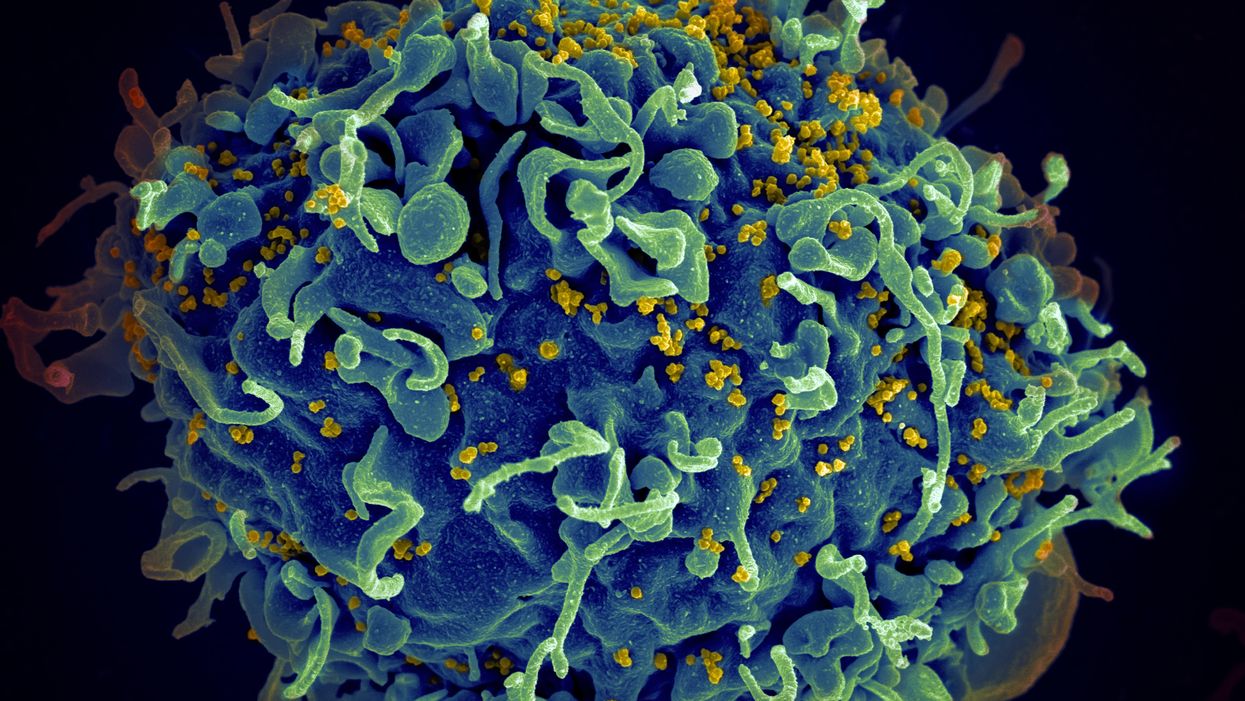
The HIV virus (yellow) infecting a human cell.
Few vaccines have been as complicated—and filled with false starts and crushed hopes—as the development of an HIV vaccine.
While antivirals help HIV-positive patients live longer and reduce viral transmission to virtually nil, these medications must be taken for life, and preventative medications like pre-exposure prophylaxis, known as PrEP, need to be taken every day to be effective. Vaccines, even if they need boosters, would make prevention much easier.
In August, Moderna began human trials for two HIV vaccine candidates based on messenger RNA.
As they have with the Covid-19 pandemic, mRNA vaccines could change the game. The technology could be applied for gene editing therapy, cancer, other infectious diseases—even a universal influenza vaccine.
In the past, three other mRNA vaccines completed phase-2 trials without success. But the easily customizable platforms mean the vaccines can be tweaked better to target HIV as researchers learn more.
Ever since HIV was discovered as the virus causing AIDS, researchers have been searching for a vaccine. But the decades-long journey has so far been fruitless; while some vaccine candidates showed promise in early trials, none of them have worked well among later-stage clinical trials.
There are two main reasons for this: HIV evolves incredibly quickly, and the structure of the virus makes it very difficult to neutralize with antibodies.
"We in HIV medicine have been desperate to find a vaccine that has effectiveness, but this goal has been elusive so far."
"You know the panic that goes on when a new coronavirus variant surfaces?" asked John Moore, professor of microbiology and immunology at Weill Cornell Medicine who has researched HIV vaccines for 25 years. "With HIV, that kind of variation [happens] pretty much every day in everybody who's infected. It's just orders of magnitude more variable a virus."
Vaccines like these usually work by imitating the outer layer of a virus to teach cells how to recognize and fight off the real thing off before it enters the cell. "If you can prevent landing, you can essentially keep the virus out of the cell," said Larry Corey, the former president and director of the Fred Hutchinson Cancer Research Center who helped run a recent trial of a Johnson & Johnson HIV vaccine candidate, which failed its first efficacy trial.
Like the coronavirus, HIV also has a spike protein with a receptor-binding domain—what Moore calls "the notorious RBD"—that could be neutralized with antibodies. But while that target sticks out like a sore thumb in a virus like SARS-CoV-2, in HIV it's buried under a dense shield. That's not the only target for neutralizing the virus, but all of the targets evolve rapidly and are difficult to reach.
"We understand these targets. We know where they are. But it's still proving incredibly difficult to raise antibodies against them by vaccination," Moore said.
In fact, mRNA vaccines for HIV have been under development for years. The Covid vaccines were built on decades of that research. But it's not as simple as building on this momentum, because of how much more complicated HIV is than SARS-CoV-2, researchers said.
"They haven't succeeded because they were not designed appropriately and haven't been able to induce what is necessary for them to induce," Moore said. "The mRNA technology will enable you to produce a lot of antibodies to the HIV envelope, but if they're the wrong antibodies that doesn't solve the problem."
Part of the problem is that the HIV vaccines have to perform better than our own immune systems. Many vaccines are created by imitating how our bodies overcome an infection, but that doesn't happen with HIV. Once you have the virus, you can't fight it off on your own.
"The human immune system actually does not know how to innately cure HIV," Corey said. "We needed to improve upon the human immune system to make it quicker… with Covid. But we have to actually be better than the human immune system" with HIV.
But in the past few years, there have been impressive leaps in understanding how an HIV vaccine might work. Scientists have known for decades that neutralizing antibodies are key for a vaccine. But in 2010 or so, they were able to mimic the HIV spike and understand how antibodies need to disable the virus. "It helps us understand the nature of the problem, but doesn't instantly solve the problem," Moore said. "Without neutralizing antibodies, you don't have a chance."
Because the vaccines need to induce broadly neutralizing antibodies, and because it's very difficult to neutralize the highly variable HIV, any vaccine will likely be a series of shots that teach the immune system to be on the lookout for a variety of potential attacks.
"Each dose is going to have to have a different purpose," Corey said. "And we hope by the end of the third or fourth dose, we will achieve the level of neutralization that we want."
That's not ideal, because each individual component has to be made and tested—and four shots make the vaccine harder to administer.
"You wouldn't even be going down that route, if there was a better alternative," Moore said. "But there isn't a better alternative."
The mRNA platform is exciting because it is easily customizable, which is especially important in fighting against a shapeshifting, complicated virus. And the mRNA platform has shown itself, in the Covid pandemic, to be safe and quick to make. Effective Covid vaccines were comparatively easy to develop, since the coronavirus is easier to battle than HIV. But companies like Moderna are capitalizing on their success to launch other mRNA therapeutics and vaccines, including the HIV trial.
"You can make the vaccine in two months, three months, in a research lab, and not a year—and the cost of that is really less," Corey said. "It gives us a chance to try many more options, if we've got a good response."
In a trial on macaque monkeys, the Moderna vaccine reduced the chances of infection by 85 percent. "The mRNA platform represents a very promising approach for the development of an HIV vaccine in the future," said Dr. Peng Zhang, who is helping lead the trial at the National Institute of Allergy and Infectious Diseases.
Moderna's trial in humans represents "a very exciting possibility for the prevention of HIV infection," Dr. Monica Gandhi, director of the UCSF-Gladstone Center for AIDS Research, said in an email. "We in HIV medicine have been desperate to find a vaccine that has effectiveness, but this goal has been elusive so far."
If a successful HIV vaccine is developed, the series of shots could include an mRNA shot that primes the immune system, followed by protein subunits that generate the necessary antibodies, Moore said.
"I think it's the only thing that's worth doing," he said. "Without something complicated like that, you have no chance of inducing broadly neutralizing antibodies."
"I can't guarantee you that's going to work," Moore added. "It may completely fail. But at least it's got some science behind it."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.
Urinary tract infections account for more than 8 million trips to the doctor each year.
Few things are more painful than a urinary tract infection (UTI). Common in men and women, these infections account for more than 8 million trips to the doctor each year and can cause an array of uncomfortable symptoms, from a burning feeling during urination to fever, vomiting, and chills. For an unlucky few, UTIs can be chronic—meaning that, despite treatment, they just keep coming back.
But new research, presented at the European Association of Urology (EAU) Congress in Paris this week, brings some hope to people who suffer from UTIs.
Clinicians from the Royal Berkshire Hospital presented the results of a long-term, nine-year clinical trial where 89 men and women who suffered from recurrent UTIs were given an oral vaccine called MV140, designed to prevent the infections. Every day for three months, the participants were given two sprays of the vaccine (flavored to taste like pineapple) and then followed over the course of nine years. Clinicians analyzed medical records and asked the study participants about symptoms to check whether any experienced UTIs or had any adverse reactions from taking the vaccine.
The results showed that across nine years, 48 of the participants (about 54%) remained completely infection-free. On average, the study participants remained infection free for 54.7 months—four and a half years.
“While we need to be pragmatic, this vaccine is a potential breakthrough in preventing UTIs and could offer a safe and effective alternative to conventional treatments,” said Gernot Bonita, Professor of Urology at the Alta Bro Medical Centre for Urology in Switzerland, who is also the EAU Chairman of Guidelines on Urological Infections.
The news comes as a relief not only for people who suffer chronic UTIs, but also to doctors who have seen an uptick in antibiotic-resistant UTIs in the past several years. Because UTIs usually require antibiotics, patients run the risk of developing a resistance to the antibiotics, making infections more difficult to treat. A preventative vaccine could mean less infections, less antibiotics, and less drug resistance overall.
“Many of our participants told us that having the vaccine restored their quality of life,” said Dr. Bob Yang, Consultant Urologist at the Royal Berkshire NHS Foundation Trust, who helped lead the research. “While we’re yet to look at the effect of this vaccine in different patient groups, this follow-up data suggests it could be a game-changer for UTI prevention if it’s offered widely, reducing the need for antibiotic treatments.”

