A Single Blood Test May Soon Replace Your Annual Physical
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
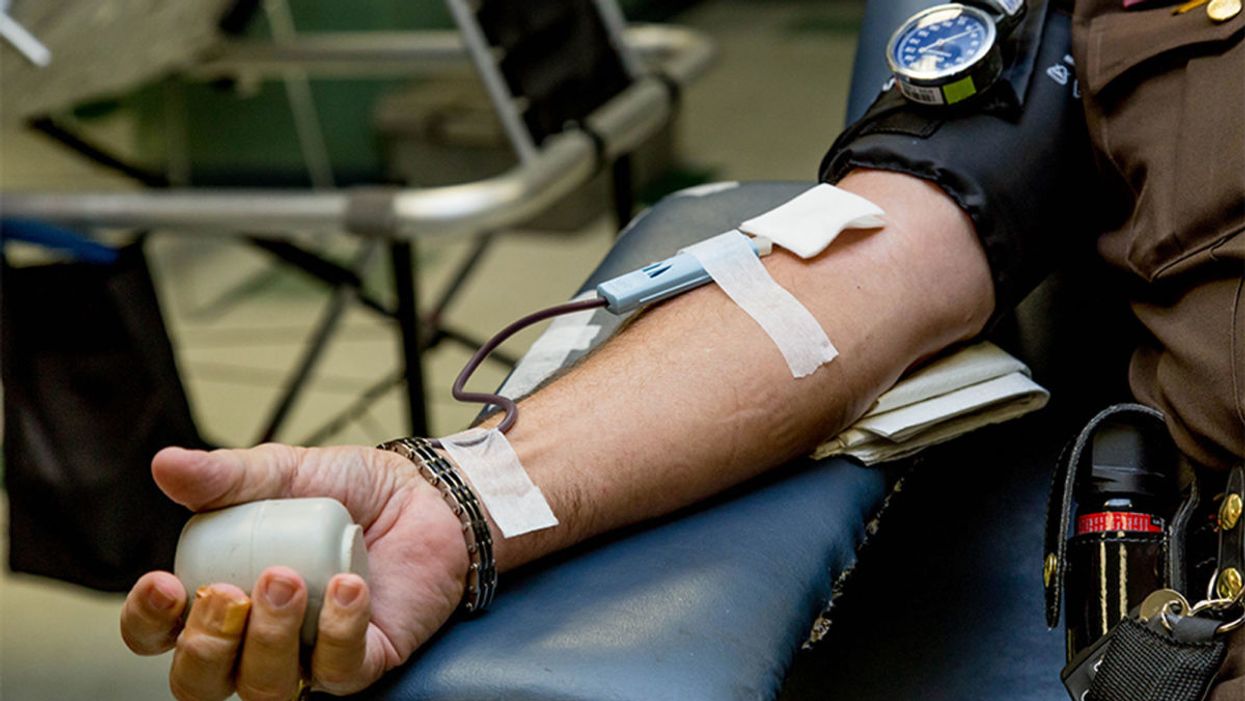
The measurement of proteins in one blood test could provide a comprehensive snapshot of a person's health in the near future.
For all the excitement over "personalized medicine" in the last two decades, its promise has not fully come to pass. Consider your standard annual physical.
Scientists have measured thousands of proteins from a single blood test to assess many individualized health conditions at once.
Your doctor still does a blood test to check your cholesterol and gauge your risk for heart disease by considering traditional risk factors (like smoking, diabetes, blood pressure) — an evaluation that has not changed in decades.
But a high-risk number alone is not enough to tell accurately whether you will suffer from heart disease. It just reflects your risk compared to population-level averages. In other words, not every person with elevated "bad" cholesterol will have a heart attack, so how can doctors determine who truly needs to give up the cheeseburgers and who doesn't?
Now, an emerging area of research may unlock some real-time answers. For the first time, as reported in the journal Nature Medicine last week, scientists have measured thousands of proteins from a single blood test to assess many individualized health conditions at once, including liver and kidney function, diabetes risk, body fat, cardiopulmonary fitness, and even smoking and alcohol consumption. Proteins can give a clear snapshot of how your body is faring at any given moment, as well as a sneak preview at what diseases may be lurking under the surface.
"Years from now," says study co-author Peter Ganz of UCSF, "we will probably be looking back on this paper as a milestone in personalized medicine."
We spoke to Ganz about the significance of this milestone. Our interview has been edited and condensed.
Is this the first study of its kind?
Yes, it is. This is a study where we measured 5,000 proteins at once to look for patterns that could either predict the risk of future diseases or inform the current state of health. Previous to this, people have measured typically one protein at a time, and some of these individual proteins have made it into clinical practice.
An example would be a protein called C-reactive protein, which is a measure of inflammation and is used sometimes in cardiology to predict the risk of future heart attacks. But what's really new is this scale. We wanted to get away from just focusing on one problem that the patient may have at a time, whether it's heart disease or kidney disease, and by measuring a much greater number of proteins, the hope is that we could inform the health of ultimately just about every organ in the body or every tissue. It's a step forward for what I would call "a one-stop shop."
"I'm very excited about personalized medicine through proteins as opposed to genes because you get both the nature and nurture."
Three things get me excited about this. One is the convenience for the patient of a single test to determine many different diseases. The second thing is the healthcare cost savings. We estimated what the cost would be to get these 11 healthcare measures that we reported on using traditional testing and the cost was upwards of 3,000 British pounds. And even though I don't know for sure what the cost of the protein tests would ultimately be, [it could come down to about $50 to $100].
The last thing is that the measurement of proteins is part of what people have called personalized medicine or precision medicine. If you look at risk factors across the population, it may not apply to individuals. In contrast, proteins are downstream of risk factors. So proteins actually tell us whether the traditional risk factors have set in motion the necessary machinery to cause disease. Proteins are the worker bees that regulate what the human body does, and so if you can find some anomalies in the proteins, that may inform us if a disease is likely to be ongoing even in its earliest stages.
Does protein testing have advantages over genetic testing for predicting future health risks?
The problem with genomics is that genes usually don't take care of the environment. It's a blueprint, but your blueprint has no idea what you will be exposed to during your lifetime in terms of the environment and lifestyle that you may choose and medications that you may be on. These are things that proteins can account for. I'm very excited about personalized medicine through proteins as opposed to genes because you get both the nature and nurture as opposed to genomics, which only gives you nature but doesn't account for anything else.
Proteins can also be tracked over time and that's not something you can do with genes. So if your behavior improves, your genes won't change, but your proteins will.
Could this new test become a regular feature of your annual physical?
That's the idea. This would be basically almost a standalone test that you could have done every year. And hopefully you wouldn't need other tests to complement this. This could be your yearly physical.
How much more does it need to be validated before it can enter the clinic and patients can trust the results?
This was a proof-of concept study. To really make this useful, we need to expand from 11 measures of health to a hundred or more health insights, to cover the whole body. And we need to expand this to all racial groups. Three of the five centers in the study were European – all Caucasian – so it's one of our high priorities to find groups of patients with better representation of minorities.
When do you expect doctors to be routinely giving this test to patients?
Much closer to five years than 20 years. We're scaling up from 11 disease states to 100, and many of those studies are underway. Results should be done within three to five years.
Do you think insurance will cover it?
Good question. I have been approached by an insurance company that wanted to understand the product better – a major insurer, with the possibility that this could actually be cost saving.
I have to ask you a curveball -- do you think that the downfall of Theranos will make consumers hesitant to trust a new technology that relies on using a single blood sample to screen for multiple health risks?
[Laughs] You're not the first person to ask me that today. I actually got a call from Elizabeth Holmes [in 2008 when I was at Harvard]. I met with her for an afternoon and met her team two more times. I gave them advice that they completely disregarded.
In many ways, what we do is diametrically opposite to Theranos. They had a culture of secrecy, and what we do is about openness. We publish, like this paper in Nature Medicine, to show the scientific details. Our supplement is much longer than the typical academic paper. We reveal everything we know. A lot of the research we do is funded by [the National Institutes of Health], and they have strict expectations about data sharing. So we agree to make the data available on a public website. If there is something we haven't done with the data, others can do it.
So you're saying that this is not another Theranos.
No, God forbid. We hope to be the opposite.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.
Urinary tract infections account for more than 8 million trips to the doctor each year.
Few things are more painful than a urinary tract infection (UTI). Common in men and women, these infections account for more than 8 million trips to the doctor each year and can cause an array of uncomfortable symptoms, from a burning feeling during urination to fever, vomiting, and chills. For an unlucky few, UTIs can be chronic—meaning that, despite treatment, they just keep coming back.
But new research, presented at the European Association of Urology (EAU) Congress in Paris this week, brings some hope to people who suffer from UTIs.
Clinicians from the Royal Berkshire Hospital presented the results of a long-term, nine-year clinical trial where 89 men and women who suffered from recurrent UTIs were given an oral vaccine called MV140, designed to prevent the infections. Every day for three months, the participants were given two sprays of the vaccine (flavored to taste like pineapple) and then followed over the course of nine years. Clinicians analyzed medical records and asked the study participants about symptoms to check whether any experienced UTIs or had any adverse reactions from taking the vaccine.
The results showed that across nine years, 48 of the participants (about 54%) remained completely infection-free. On average, the study participants remained infection free for 54.7 months—four and a half years.
“While we need to be pragmatic, this vaccine is a potential breakthrough in preventing UTIs and could offer a safe and effective alternative to conventional treatments,” said Gernot Bonita, Professor of Urology at the Alta Bro Medical Centre for Urology in Switzerland, who is also the EAU Chairman of Guidelines on Urological Infections.
The news comes as a relief not only for people who suffer chronic UTIs, but also to doctors who have seen an uptick in antibiotic-resistant UTIs in the past several years. Because UTIs usually require antibiotics, patients run the risk of developing a resistance to the antibiotics, making infections more difficult to treat. A preventative vaccine could mean less infections, less antibiotics, and less drug resistance overall.
“Many of our participants told us that having the vaccine restored their quality of life,” said Dr. Bob Yang, Consultant Urologist at the Royal Berkshire NHS Foundation Trust, who helped lead the research. “While we’re yet to look at the effect of this vaccine in different patient groups, this follow-up data suggests it could be a game-changer for UTI prevention if it’s offered widely, reducing the need for antibiotic treatments.”

